Are you asking for 'how to write a net ionic equation'? Here you can find questions and answers on the topic.
Table of contents
- How to write a net ionic equation in 2021
- How to write ionic equations
- Net ionic reaction calculator
- Balanced net ionic equation calculator
- How to find net ionic equation calculator
- Ionic formula calculator
- How to write a net ionic equation for a double replacement reaction
- How to write a net ionic equation with no precipitate
How to write a net ionic equation in 2021
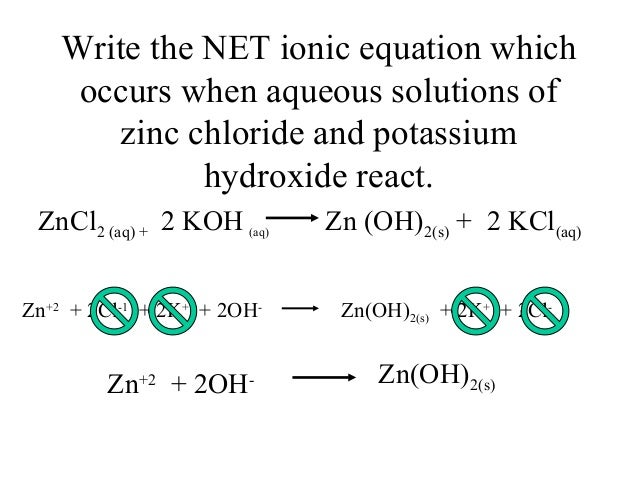 This picture shows how to write a net ionic equation.
This picture shows how to write a net ionic equation.
How to write ionic equations
 This image representes How to write ionic equations.
This image representes How to write ionic equations.
Net ionic reaction calculator
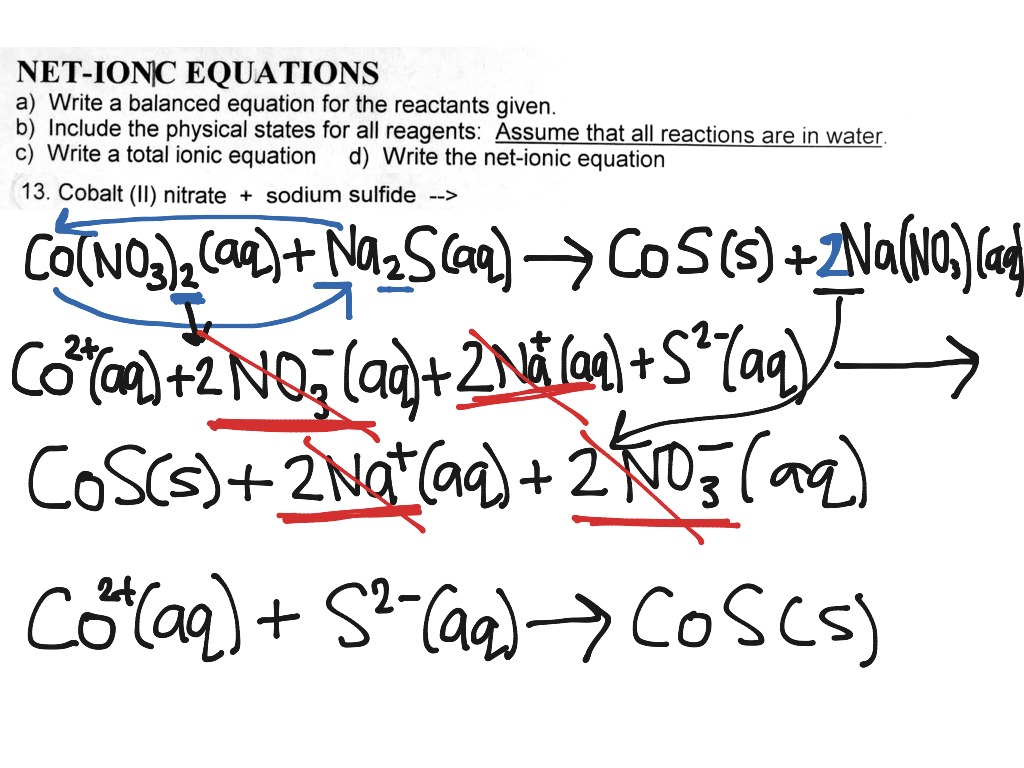 This picture representes Net ionic reaction calculator.
This picture representes Net ionic reaction calculator.
Balanced net ionic equation calculator
 This image representes Balanced net ionic equation calculator.
This image representes Balanced net ionic equation calculator.
How to find net ionic equation calculator
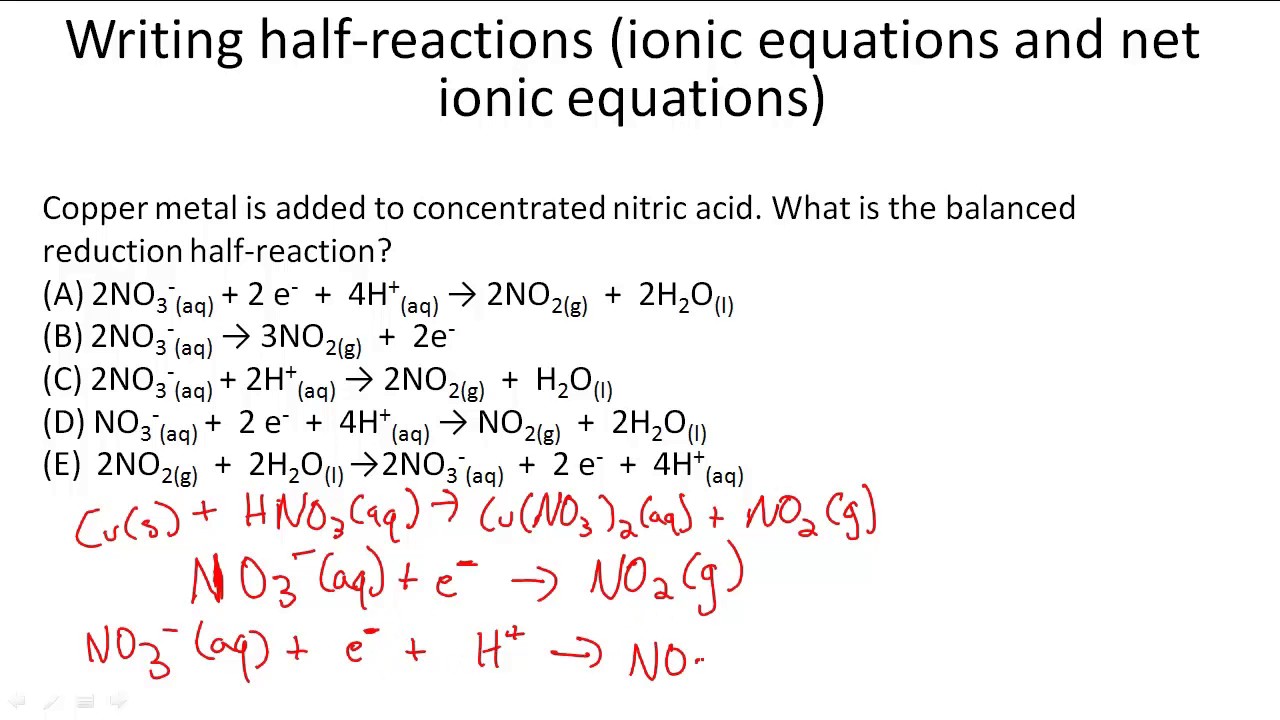 This picture shows How to find net ionic equation calculator.
This picture shows How to find net ionic equation calculator.
Ionic formula calculator
 This picture representes Ionic formula calculator.
This picture representes Ionic formula calculator.
How to write a net ionic equation for a double replacement reaction
 This image illustrates How to write a net ionic equation for a double replacement reaction.
This image illustrates How to write a net ionic equation for a double replacement reaction.
How to write a net ionic equation with no precipitate
 This image illustrates How to write a net ionic equation with no precipitate.
This image illustrates How to write a net ionic equation with no precipitate.
When do you write a net ionic equation?
When ions are involved in a reaction, the equation for the reaction can be written with various levels of detail. Depending on which part of the reaction you are interested in, you might write a molecular, complete ionic, or net ionic equation.
Why are there six ions on reactant side of net ionic equation?
For example, there are six chloride ions on the reactant side because the coefficient of 3 is multiplied by the subscript of 2 in the copper (II) chloride formula. The spectator ions are K + and Cl − and can be eliminated. For a precipitation reaction, the net ionic equation always shows the two ions that come together to form the precipitate.
How are ionic equations similar to chemical equations?
Writing ionic equation is extremely similar to writing chemical equations. Recall that ionic compounds that dissolved in water will dissociate completely into ions (have charge). In an ionic equation: Number of atoms of each elements must be balanced. Total charges carried by the ions must be balanced (e.g. +3 on left must have +3 on right as well)
Why are spectator ions omitted from the ionic equation?
Recall that ionic compounds that dissolved in water will dissociate completely into ions (have charge). Total charges carried by the ions must be balanced (e.g. +3 on left must have +3 on right as well) Spectator ions are omitted from the ionic equation. (Spectator ions are ions that do not take part in the reaction)
Last Update: Oct 2021